Understanding The Periodic Trends In The Periodic Table: A Comprehensive Guide
Understanding the Periodic Trends in the Periodic Table: A Comprehensive Guide
Understanding the Periodic Trends in the Periodic Table: A Comprehensive Guide
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to Understanding the Periodic Trends in the Periodic Table: A Comprehensive Guide. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
- 1 Understanding the Periodic Trends in the Periodic Table: A Comprehensive Guide
- 2 Introduction
- 3 Understanding the Periodic Trends in the Periodic Table: A Comprehensive Guide
- 3.1 Periodic Trends: A Foundation for Understanding Chemistry
- 3.2 Applications and Importance of Periodic Trends
- 3.3 Related Searches:
- 3.4 FAQs:
- 3.5 Tips for Studying Periodic Trends:
- 3.6 Conclusion:
- 4 Closure
Understanding the Periodic Trends in the Periodic Table: A Comprehensive Guide
/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)
The periodic table is a fundamental tool in chemistry, organizing elements based on their atomic structure and resulting properties. This organization reveals recurring patterns, known as periodic trends, which provide valuable insights into the behavior of elements and their interactions.
Periodic Trends: A Foundation for Understanding Chemistry
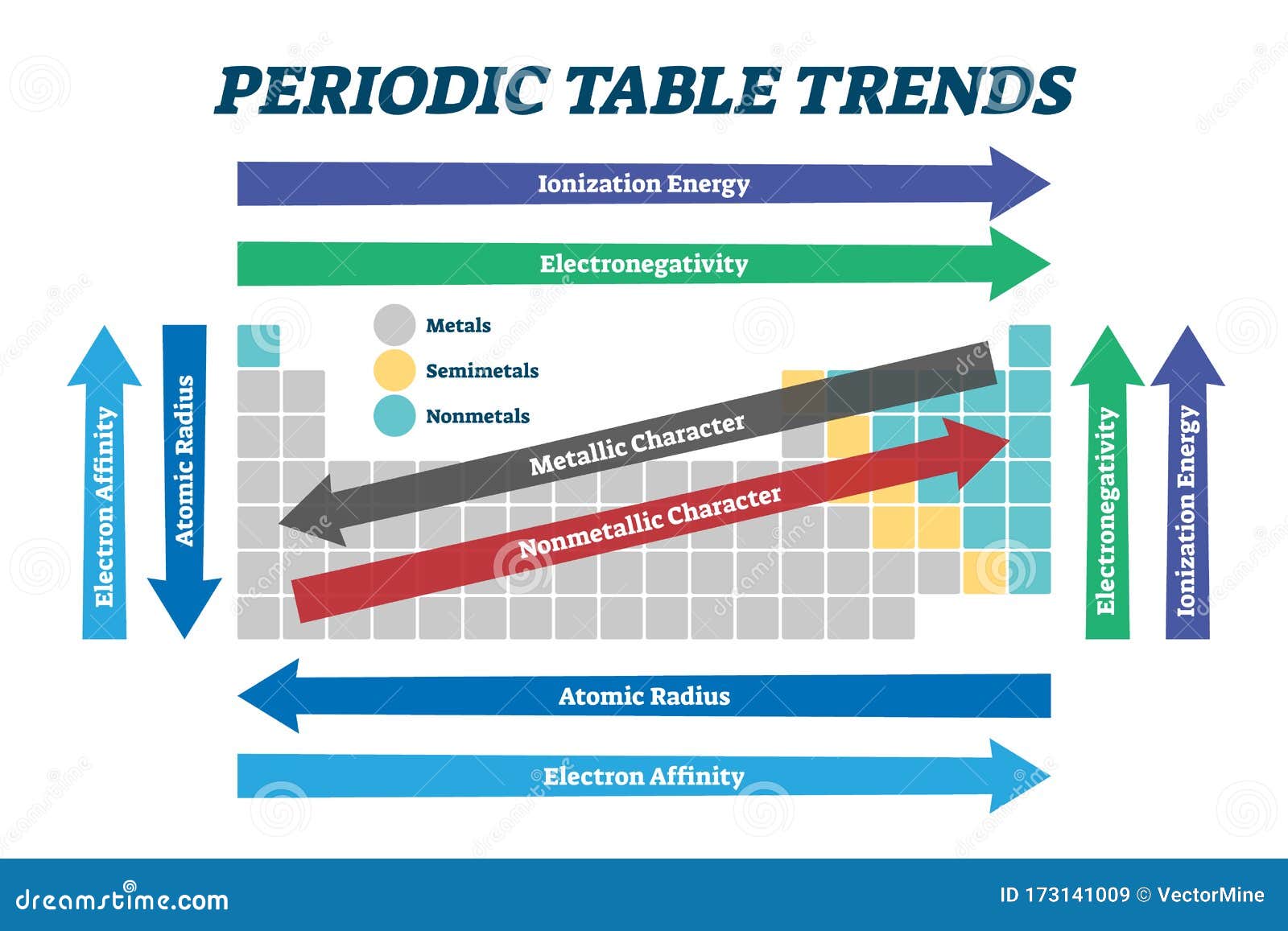
Periodic trends are the predictable and systematic changes in the chemical and physical properties of elements as one moves across a period (horizontal row) or down a group (vertical column) of the periodic table. These trends arise from the changing arrangement of electrons in atoms, specifically the valence electrons, which are those in the outermost shell.
Here are the key periodic trends and their explanations:
-
Atomic Radius: The atomic radius is the distance from the nucleus to the outermost electron shell. It generally decreases across a period and increases down a group.
- Across a period: As you move across a period, the number of protons in the nucleus increases, leading to a stronger attraction between the nucleus and electrons. This pulls the electrons closer to the nucleus, decreasing the atomic radius.
- Down a group: As you move down a group, the number of electron shells increases. The outermost electrons are farther from the nucleus, leading to a larger atomic radius.
-
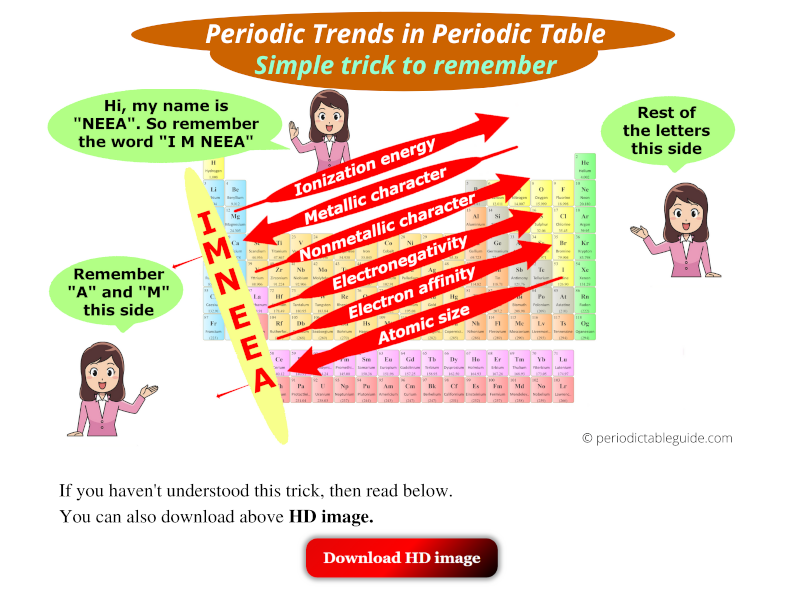
Ionization Energy: Ionization energy is the minimum energy required to remove an electron from a gaseous atom in its ground state. It generally increases across a period and decreases down a group.
- Across a period: The increasing nuclear charge across a period makes it more difficult to remove an electron, leading to higher ionization energy.
- Down a group: The increasing distance between the nucleus and the outermost electron makes it easier to remove an electron, leading to lower ionization energy.
-
Electron Affinity: Electron affinity is the change in energy when an electron is added to a neutral atom in its gaseous state to form a negative ion. It generally increases across a period and decreases down a group.
- Across a period: The increasing nuclear charge across a period attracts incoming electrons more strongly, leading to a more negative electron affinity (more energy released).
- Down a group: The increasing distance between the nucleus and the outermost electron weakens the attraction for an incoming electron, leading to a less negative electron affinity (less energy released).
-
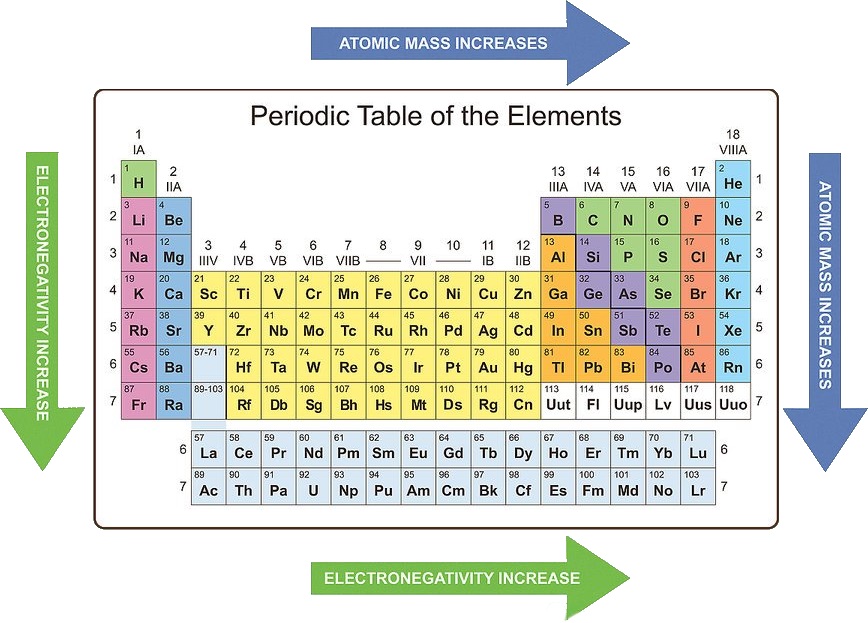
Electronegativity: Electronegativity is the measure of an atom’s ability to attract electrons in a chemical bond. It generally increases across a period and decreases down a group.
- Across a period: The increasing nuclear charge across a period makes the atom more attractive to electrons, leading to higher electronegativity.
- Down a group: The increasing distance between the nucleus and the outermost electron weakens the attraction for electrons, leading to lower electronegativity.
Applications and Importance of Periodic Trends
Periodic trends have numerous applications in various fields:
- Predicting Chemical Properties: Understanding periodic trends allows chemists to predict the reactivity and bonding behavior of elements. This knowledge is crucial for designing new materials, synthesizing novel compounds, and understanding chemical reactions.
- Developing New Technologies: The ability to predict element properties based on their position in the periodic table is essential for developing new technologies. For example, understanding the ionization energy of elements helps in designing semiconductors for electronics.
- Environmental Applications: Periodic trends are vital in understanding the environmental impact of elements. For instance, knowing the electronegativity of elements helps in predicting the toxicity of pollutants.
- Understanding Biological Systems: Periodic trends play a crucial role in understanding the behavior of elements in biological systems. For example, the electronegativity of elements influences the formation of biomolecules like proteins and nucleic acids.
Related Searches:
1. Periodic Table Trends and Reactivity:
The reactivity of an element is directly related to its ability to gain or lose electrons. Understanding periodic trends in ionization energy and electron affinity allows us to predict the reactivity of elements. For instance, elements with low ionization energy readily lose electrons and are highly reactive metals, while elements with high electron affinity readily gain electrons and are highly reactive nonmetals.
2. Periodic Table Trends and Bonding:
The type of chemical bond formed between atoms depends on the electronegativity difference between them. Periodic trends in electronegativity allow us to predict the type of bond formed, whether ionic (large electronegativity difference) or covalent (small electronegativity difference).
3. Periodic Table Trends and Metallic Character:
Metallic character refers to the tendency of an element to lose electrons and form positive ions. It generally increases down a group and decreases across a period. This trend is explained by the increasing distance between the nucleus and the outermost electron down a group, making it easier to lose electrons.
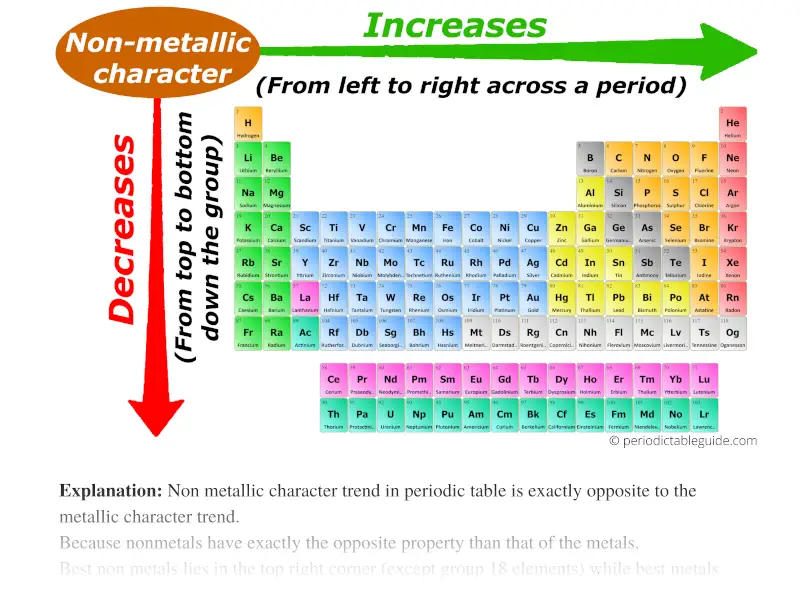
4. Periodic Table Trends and Nonmetallic Character:
Nonmetallic character refers to the tendency of an element to gain electrons and form negative ions. It generally decreases down a group and increases across a period. This trend is explained by the increasing nuclear charge across a period, making the atom more attractive to electrons.
5. Periodic Table Trends and Melting Point:
The melting point of an element is the temperature at which it transitions from a solid to a liquid. While there are exceptions, generally, melting point increases down a group due to stronger metallic bonding and decreases across a period due to weaker interatomic forces.
6. Periodic Table Trends and Boiling Point:
The boiling point of an element is the temperature at which it transitions from a liquid to a gas. Similar to melting point, boiling point generally increases down a group due to stronger intermolecular forces and decreases across a period due to weaker intermolecular forces.
7. Periodic Table Trends and Conductivity:
Conductivity refers to the ability of a material to conduct electricity or heat. Generally, metals are good conductors due to the presence of free electrons, while nonmetals are poor conductors. This trend is related to the ease with which electrons can move through the material.
8. Periodic Table Trends and Density:
Density is the mass per unit volume of a substance. Density generally increases across a period due to increasing atomic mass and decreases down a group due to increasing atomic volume.
FAQs:
Q: What is the importance of understanding periodic trends?
A: Understanding periodic trends is crucial for predicting the behavior of elements and their interactions, leading to advancements in various fields like chemistry, material science, technology, and environmental science.
Q: How do periodic trends affect the reactivity of elements?
A: Elements with low ionization energy readily lose electrons and are highly reactive metals, while elements with high electron affinity readily gain electrons and are highly reactive nonmetals.
Q: How do periodic trends affect the type of chemical bond formed?
A: The electronegativity difference between atoms determines the type of bond formed. A large electronegativity difference leads to an ionic bond, while a small difference leads to a covalent bond.
Q: How do periodic trends affect the metallic and nonmetallic character of elements?
A: Metallic character increases down a group and decreases across a period, while nonmetallic character decreases down a group and increases across a period.
Q: How do periodic trends affect the melting point and boiling point of elements?
A: Generally, melting point and boiling point increase down a group and decrease across a period.
Q: How do periodic trends affect the conductivity and density of elements?
A: Metals are good conductors, while nonmetals are poor conductors. Density generally increases across a period and decreases down a group.
Tips for Studying Periodic Trends:
- Visualize the Periodic Table: Familiarize yourself with the layout of the periodic table and the location of different groups and periods.
- Use Mnemonic Devices: Create mnemonic devices to remember the trends, such as "Across a period, ionization energy goes up, down a group, it goes down."
- Practice Problems: Solve practice problems to reinforce your understanding of periodic trends and their applications.
- Connect Trends to Real-World Examples: Relate periodic trends to real-world examples, such as the use of metals in electronics or the reactivity of alkali metals in water.
- Utilize Online Resources: Explore online resources like interactive periodic tables and simulations to visualize and understand periodic trends.
Conclusion:
Periodic trends are fundamental concepts in chemistry that provide valuable insights into the behavior of elements. Understanding these trends allows us to predict the properties of elements, design new materials, develop technologies, and address environmental concerns. By studying and applying periodic trends, we gain a deeper understanding of the world around us.
.PNG)
/periodictrendstable-5c4a46614cedfd000187c5db.jpg)

Closure
Thus, we hope this article has provided valuable insights into Understanding the Periodic Trends in the Periodic Table: A Comprehensive Guide. We thank you for taking the time to read this article. See you in our next article!