Understanding Periodic Trends: A Comprehensive Guide
Understanding Periodic Trends: A Comprehensive Guide
Understanding Periodic Trends: A Comprehensive Guide
Introduction
With great pleasure, we will explore the intriguing topic related to Understanding Periodic Trends: A Comprehensive Guide. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Understanding Periodic Trends: A Comprehensive Guide
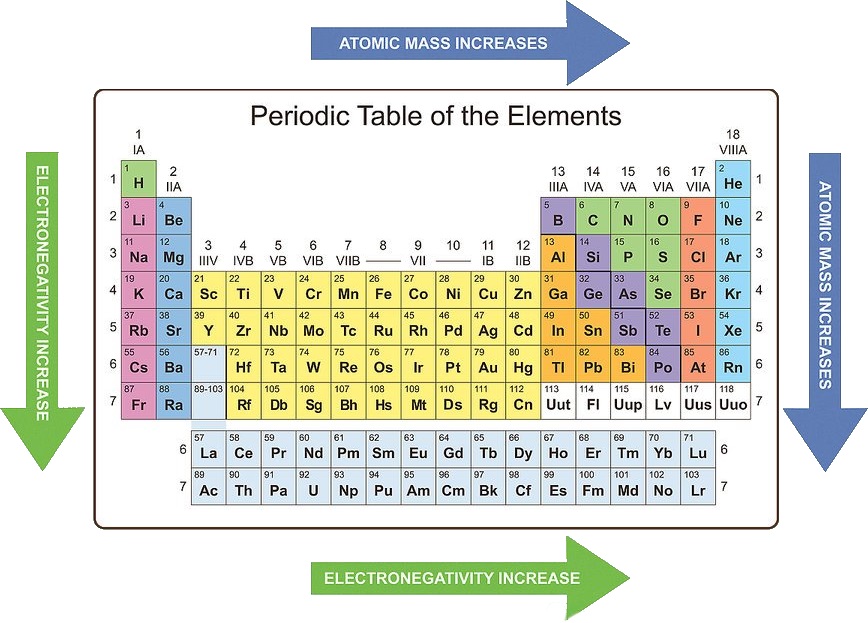
The periodic table, a fundamental tool in chemistry, organizes elements based on their atomic number and recurring chemical properties. These recurring patterns, known as periodic trends, provide valuable insights into the behavior of elements and their interactions. This guide will delve into the key periodic trends, explaining their significance and exploring their application in various chemical phenomena.
Key Periodic Trends
-
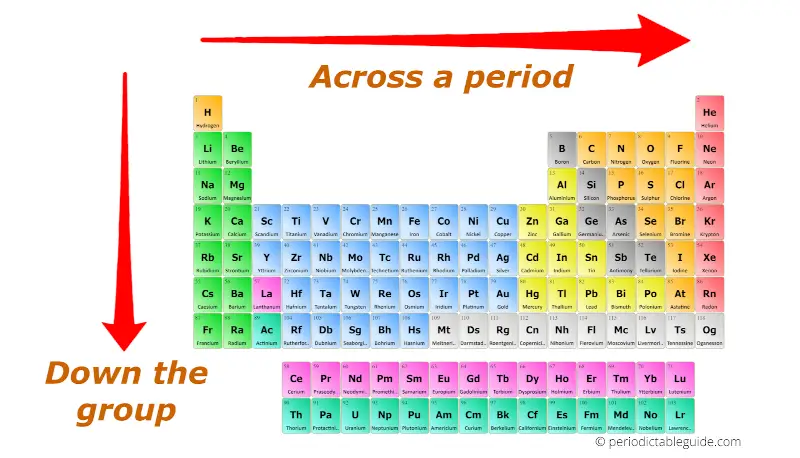
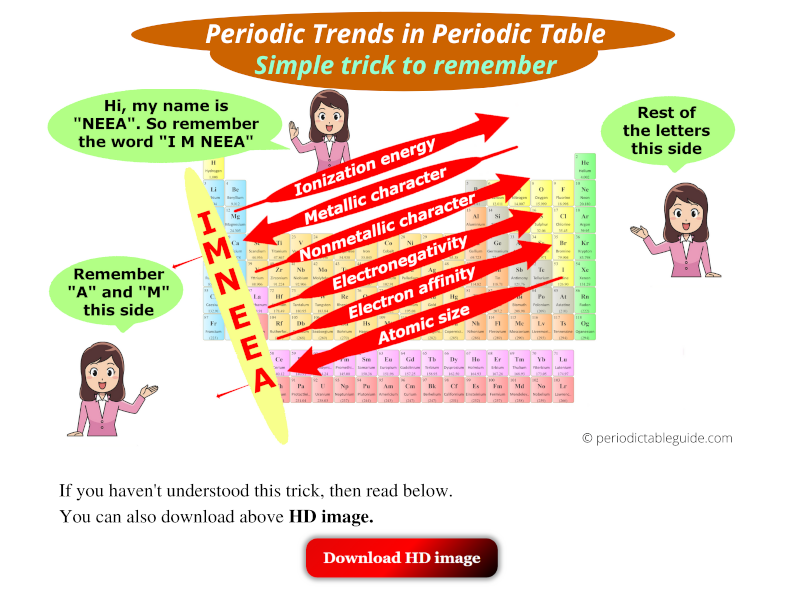
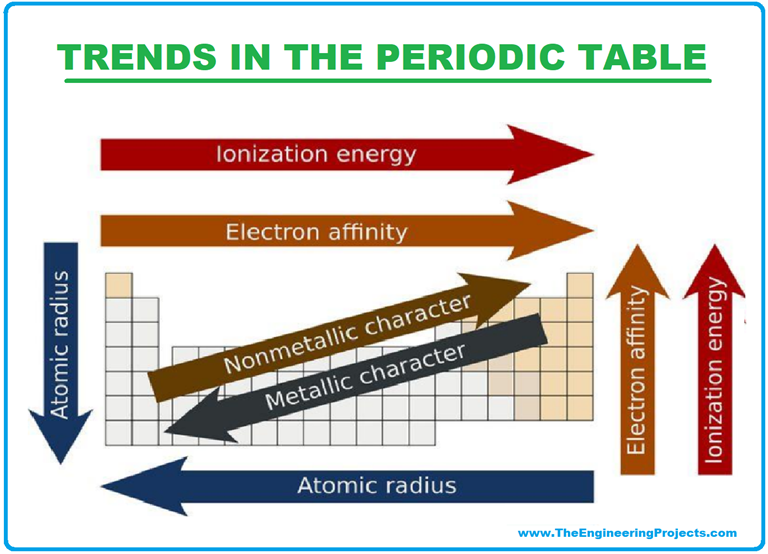
Atomic Radius: The atomic radius refers to the distance between the nucleus of an atom and its outermost electron shell. Moving across a period (from left to right), the atomic radius generally decreases due to increasing nuclear charge, which attracts electrons more strongly, pulling them closer to the nucleus. Conversely, moving down a group (from top to bottom), the atomic radius increases as additional electron shells are added, leading to greater distance between the nucleus and outermost electrons.
-
Ionization Energy: Ionization energy represents the minimum energy required to remove an electron from a gaseous atom in its ground state. Ionization energy generally increases across a period as the attraction between the nucleus and electrons intensifies due to increased nuclear charge. Down a group, ionization energy decreases because the outermost electrons are further from the nucleus and experience weaker attraction.
-
Electron Affinity: Electron affinity measures the change in energy when an electron is added to a neutral atom in its gaseous state. Generally, electron affinity increases across a period as the atom’s attraction for electrons strengthens. However, down a group, electron affinity generally decreases due to the increasing distance between the nucleus and incoming electron.
-
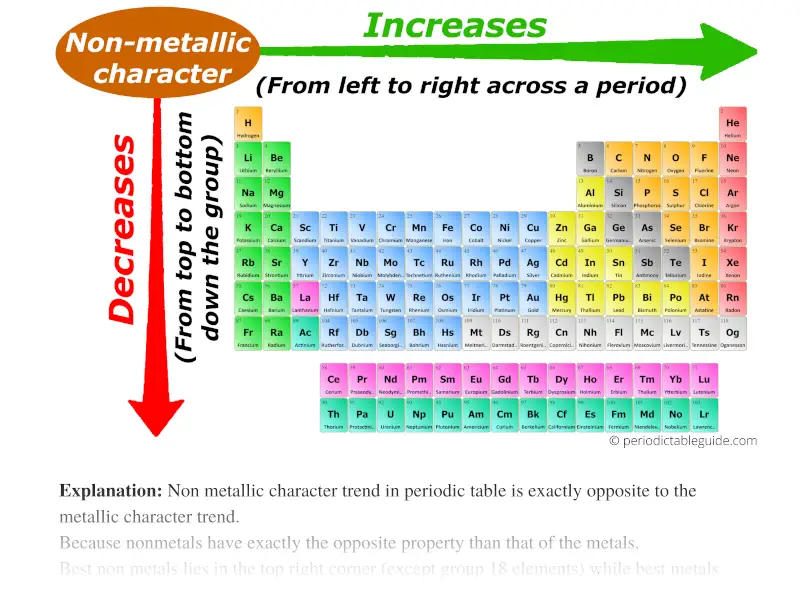
Electronegativity: Electronegativity quantifies an atom’s ability to attract electrons in a chemical bond. It generally increases across a period as the nuclear charge rises, making the atom more attractive to shared electrons. Down a group, electronegativity decreases as the outermost electrons are further from the nucleus and experience weaker attraction.
Understanding the Importance of Periodic Trends
The understanding of periodic trends is crucial for:
-
Predicting Chemical Properties: Periodic trends allow us to predict the reactivity and bonding behavior of elements based on their position on the periodic table. For example, elements with high electronegativity tend to form ionic bonds with elements with low electronegativity.
-
Explaining Chemical Reactions: Periodic trends help explain why certain reactions occur and others don’t. For instance, the high ionization energy of noble gases explains their inert nature.
-
Designing New Materials: By understanding periodic trends, scientists can design new materials with specific properties, such as high conductivity or strength.
Applications of Periodic Trends in Chemistry
-
Bonding: Periodic trends play a vital role in understanding the types of bonds formed between elements. For example, elements with high electronegativity differences tend to form ionic bonds, while elements with similar electronegativity values form covalent bonds.
-
Reactivity: Periodic trends help predict the reactivity of elements. Elements with low ionization energy are more likely to lose electrons and act as reducing agents, while elements with high electron affinity tend to gain electrons and act as oxidizing agents.
-
Acidity and Basicity: Periodic trends influence the acidity and basicity of compounds. For example, oxides of metals tend to be basic, while oxides of nonmetals are typically acidic.
Related Searches
-
Periodic Trends Chart: A visual representation of periodic trends, often depicted as graphs or tables, provides a clear understanding of the variation in properties across the periodic table.
-
Periodic Trends Worksheet: Worksheets with practice problems related to periodic trends help students apply their knowledge and reinforce their understanding.
-
Periodic Trends Examples: Real-world examples of periodic trends, such as the increasing melting point of metals down a group, provide a practical context for understanding these concepts.
-
Periodic Trends Quiz: Quizzes on periodic trends assess students’ understanding of the key concepts and their ability to apply them to various scenarios.
-
Periodic Trends and Chemical Bonding: The relationship between periodic trends and chemical bonding is crucial for understanding the formation of molecules and their properties.
-
Periodic Trends and Reactivity: Understanding how periodic trends influence the reactivity of elements is essential for predicting chemical reactions and designing new materials.
-
Periodic Trends and the Periodic Table: The periodic table serves as the foundation for understanding periodic trends, and its organization reflects the recurring patterns in elemental properties.
-
Periodic Trends and the History of Chemistry: The discovery and explanation of periodic trends have played a significant role in the development of modern chemistry.
FAQs
-
Why do atomic radii decrease across a period?
- The increasing nuclear charge across a period attracts electrons more strongly, pulling them closer to the nucleus and decreasing the atomic radius.
-
Why do ionization energies increase across a period?
- The increasing nuclear charge across a period makes it more difficult to remove an electron from the atom, resulting in higher ionization energy.
-
Why do electronegativities increase across a period?
- The increasing nuclear charge across a period makes the atom more attractive to shared electrons, leading to higher electronegativity.
-
Why do atomic radii increase down a group?
- The addition of electron shells down a group increases the distance between the nucleus and outermost electrons, leading to a larger atomic radius.
-
What are the exceptions to periodic trends?
- There are some exceptions to periodic trends, particularly in the case of small atoms and elements with partially filled d-orbitals. These exceptions can be attributed to factors like electron shielding and interelectronic repulsion.
Tips for Understanding Periodic Trends
-
Visualize the Periodic Table: Use a periodic table as a visual aid to understand the trends and their relationship to the arrangement of elements.
-
Practice with Examples: Apply your knowledge of periodic trends to real-world examples and practice problems to solidify your understanding.
-
Connect the Trends: Understand how different periodic trends are interconnected and how they influence each other.
-
Use Resources: Utilize textbooks, online resources, and educational videos to gain a deeper understanding of periodic trends.
Conclusion
Periodic trends are fundamental concepts in chemistry that provide a framework for understanding the properties and reactivity of elements. By understanding these trends, we can predict chemical behavior, design new materials, and explain various chemical phenomena. The periodic table, with its organized arrangement of elements, serves as a powerful tool for exploring and applying these trends, further enriching our understanding of the chemical world.
/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)


Closure
Thus, we hope this article has provided valuable insights into Understanding Periodic Trends: A Comprehensive Guide. We thank you for taking the time to read this article. See you in our next article!
.PNG)